Caren J. Frost, PhD, MPH
How is the HRPP related to the IRB?
As part of the HRPP, the primary goal of the Institutional Review Board (IRB) is to protect the rights and welfare of human research subjects recruited to participate in research activities conducted under the auspices of the University of Utah.
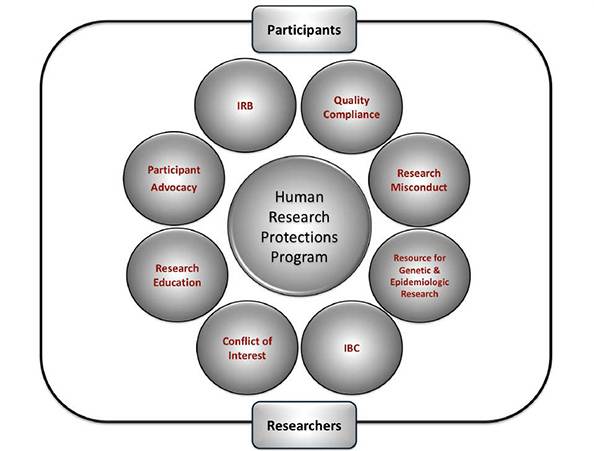
Objectives of the HRPP:
- Promote high quality ethical and transparent research activities by serving as a resource for researchers at all levels
- Advocate for the rights and welfare of participants in research programs conducted by University of Utah faculty, staff, and students.
- The units of the ORIC providing specialized ethics and training resources, as well as addressing questions about meeting institutional and federal research requirements.
Departments
Conflict of Interest
Supporting the University community in identifying and managing financial conflicts of interest in research and scholarly activities, procurement, and intellectual property.
Human Subject Research
The IRB is charged with the review of all research projects that involve humans to ensure they comply with local, state, and federal laws, as well as the high ethical standards set forth by the University.
Institutional Biosafety Committee
Providing oversight, training, services, and supplies to support research involving hazardous biological materials.
Quality Compliance
Facilitating ethical, efficient and high-quality research by protecting overall data integrity.
Research Education
Offering current education and training opportunities designed to support, develop, and maintain a standardized body of knowledge and best practice methodology for research personnel at the University of Utah.
Research Participant Advocacy
Supporting researchers and research participants with the goal of facilitating transparency, efficiency, success, and safety in research by providing language services in translation and interpretation.
Resource for Genetic & Epidemiologic Research (RGE)
Overseeing the Utah Population Data Resource for the collection, storage, study, and dissemination of medical and related information for the purpose of reducing morbidity or mortality, or for the purpose of evaluating and improving the quality of hospital and medical care.
Read more About Resource for Genetic & Epidemiologic Research (RGE)

